Endotoxemia challenge
in healthy volunteers
After approval of the local ethics committee and written informed consent, all subjects are thoroughly screened prior to inclusion (using medical history, physical examination, laboratory tests, and a 12-leads electrocardiogram). The procedure and general requirements for conducting a endotoxemia challenge are as follows:
- On the day of the experiment, subjects are hospitalized and receive one or two (18 gauge) venous catheters to accommodate infusion of endotoxin, fluids, and (if applicable) study medication
- Subsequently, an arterial catheter is placed, preferably in the radial artery of the non-dominant arm, for frequent blood withdrawals and blood pressure monitoring.
- Vital signs are continuously monitored during the experiment using 3-lead electrocardiography, peripheral pulse-oximetry, and the intra-arterial blood pressure signal.
- Body temperature (determined using an ear thermometer) and severity of symptoms are determined every half hour.
- Approximately 1 hour prior to endotoxin administration, subjects are prehydrated with 1.5 L Sodium Chloride 0.45%/Glucose 2.5% solution, as this is known to reduce the risk of a vasovagal reaction following endotoxemia. Hereafter, fluid administration is continued at a rate of 150 mL/h until the end of the experiment.
- Arterial blood is drawn at baseline and serially during the endotoxemia to determine leukocyte differentiation and cytokine concentration over time.
Depending on the research questions proposed, the evaluation of numerous parameters and/or (pharmacological) interventions can be added to the protocol.
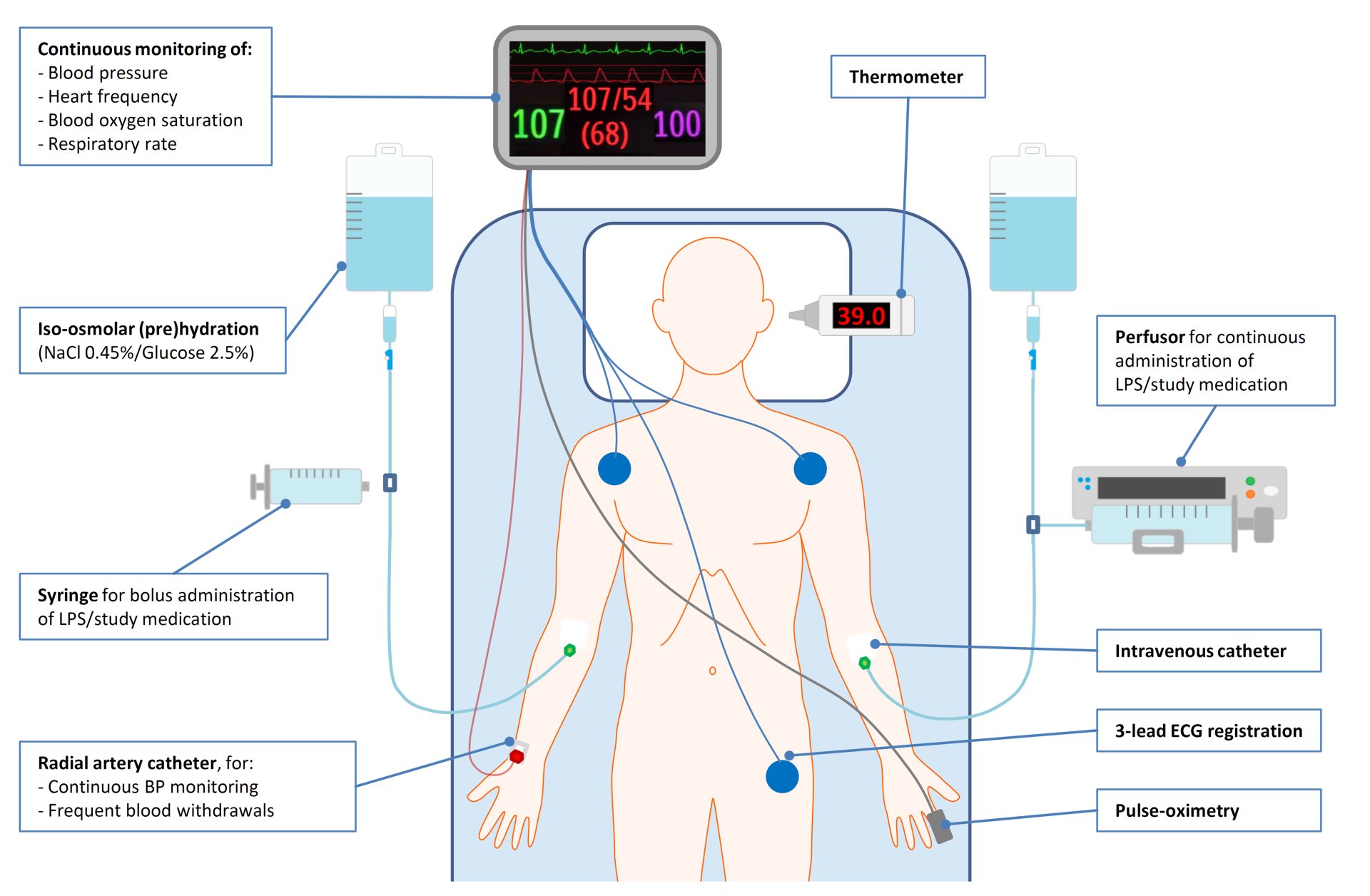 LPS = lipopolysaccharide, BP = blood pressure, ECG = electrocardiography, NaCl = Sodium chloride
LPS = lipopolysaccharide, BP = blood pressure, ECG = electrocardiography, NaCl = Sodium chloride 