
Valentina Palacio-Castañeda, theme Nanomedicine and colleagues, published in Cancers about a hybrid in silico and tumor-on-a-chip approach to model targeted protein behavior in 3D microenvironments.
Engineered proteins possess a great therapeutic potential, but their development as novel therapeutics is impeded by the lack of in vitro models that accurately mimic human physiology. Furthermore, it is often challenging to extrapolate results from animal models to the human situation, and there is an increasing societal pressure to investigate alternatives for animal research due to ethical concerns.
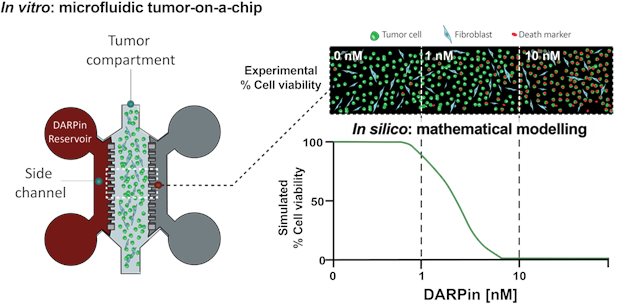
In a study conducted by Wouter Verdurmen, assistant professor, and PhD candidate Valentina Palacio-Castañeda from the Department of Biochemistry, in collaboration with researchers from the Institut Curie in Paris, an alternative approach to study therapeutic protein behavior and delivery in complex 3D microenvironments was developed. The research was published on May 18th in the special issue Cancer-on-a-Chip: Applications and Challenges from the journal Cancers.
The study employed a hybrid approach combining mathematical modeling with 3D in vitro models, including tumor spheroids and a tumor-on-a-chip device. Mathematical modeling was utilized to simulate the delivery of engineered therapeutic proteins targeting cancer cells and to predict the biological activity. By cross-comparing simulated and experimental data from 3D models, the researchers were able to correctly predict the best dose needed to deliver therapeutic proteins for eradication of tumor cells, while leaving the surrounding non-tumor cells untouched. This study shows the potential of combining computational approaches with novel in vitro models to advance the development of protein therapeutics. The group of Verdurmen focuses on investigating cytosolic delivery of engineered proteins targeted to specific cell surface receptors and uses different in vitro 3D and organ-on-a-chip platforms and in silico studies that aid in the rational development of a next generation of protein therapeutics.
Related news items

Camille Le Gall reports an efficient approach for delivery of poorly soluble tumor antigens to human cDC1s
20 April 2022 Camille Le Gall published about an efficient targeting of NY-ESO-1 tumor antigen to human cDC1s by lymphotactin results in cross-presentation and antigen-specific T cell expansion in the Journal of Immunotherapy of Cancer. go to page
Rubicon grants awarded to three RIMLS researchers
19 April 2022Three researchers have received Rubicon funding from NWO/ZonMw. This will enable Elke Muntjewerff, Laura de Vries and Laurens van de Wiel to do research at a foreign research institute for the next two years.
go to page
Large NWA ORC grant awarded for national skin research: Next Generation ImmunoDermatology
23 March 2022Research for better treatment methods for chronic skin diseases.
go to page
ERC Proof of Concept Grant for Martijn Verdoes
8 March 2022 Martijn Verdoes, group leader Chemical Immunology at the department of Tumor Immunology, has been awarded an ERC Proof of Concept (PoC) Grant. The ERC PoC Grants are designed to support ERC grantees with the commercial or societal application of the results of their funded research. go to page
NWO OTP grant to investigate fibrotic diseases with organ-on-a-chip technology
22 December 2021 Wouter Verdurmen & Peter van der Kraan have been awarded with a grant by the Netherlands Organisation for Scientific Research (NWO-OTP) to develop and employ innovative organ-on-a-chip models to investigate fibrotic diseases, with a particular focus on systemic sclerosis. go to page
