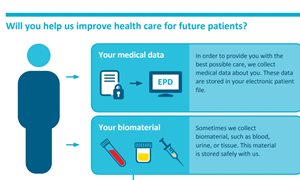
To give you the best possible treatment, we collect medical data about you. Sometimes we collect biomaterial, such as blood, urine, or tissue. We would like to use your medical data and leftover biomaterial for research and education. This is how you can help us improve care for all patients. However, we can only use your medical data and biomaterial with your consent.
What is medical data and biomaterial?
Medical data
Medical data is all information collected about you during your physical examination and treatment. We record this information in your electronic patient file. It includes:
- Information about yourself, such as your height, weight, gender, and age. Please note that you cannot be identified from this information.
- Information about your health condition or illness. This also includes your medication, treatments, and health outcomes after receiving these medications and/or treatments.
- Medical imaging, such as x-rays, ultrasounds and scans.
- Voice recordings, such as voice recordings from a visit to a speech therapist.
Biomaterial
Common biomaterials are blood, urine and tissue. Tissue is biomaterial that a doctor or nurse removes during a needle puncture, biopsy or operation. This can be healthy tissue or unhealthy tissue, such as a cancerous tumor.
Questions?

Peggy Manders
manager Radboud Biobank
(024) 365 36 78
contact form
How do you give consent?
Only you can decide if you would like to give consent. To make your choice clear to everyone, we save your answer in your patient file. read moreHow do you give consent?
Only you can decide if you would like to give consent. To make your choice clear to everyone, we save your answer in your patient file.
For new patients (from January 13, 2020)
During your registration, an assistant from the central registration desk will ask you if you would like to give your consent. If you consent, this is recorded in your patient file. If you do not want to decide right away, you can learn more about the use of medical data and leftover biomaterial in mijnRadboud or at our website. You can then give your consent in mijnRadboud or inform an assistant at the central registration desk.
For existing patients
If you are already a patient at the Radboudumc, we do not have to ask for your consent again. If you would like to withdraw your consent, you can inform an assistant at the central registration desk. You can also withdraw your consent in mijnRadboud. If you decide to withdraw your consent, this will not affect your treatment or care with us.
Do you not want to give consent?
For new patients (from January 13, 2020)
During your registration, an assistant from the central registration desk will ask you if you would like to give your consent. If you do not consent, this is recorded in your patient file. If you do not want to decide right away, you can learn more about the use of medical data and leftover biomaterial in mijnRadboud or at our website. You can then give your consent in mijnRadboud or inform an assistant at the central registration desk.
You do not have to explain why you do not consent. You will receive the same care from your doctor and healthcare providers. We will need to keep your medical information and biomaterial for your current and future treatments. However, we will not use your medical information and biomaterial for medical research.
For existing patients
If you are already a patient at the Radboudumc, we do not have to ask for your consent again. If you would like to withdraw your consent, you can inform an assistant at the central registration desk. You can also withdraw your consent in mijnRadboud. If you decide to withdraw your consent, this will not affect your treatment or care with us.
What do we want to do with your medical data and biomaterial?
We use it to conduct medical research and examine relationships between diseases and health conditions. We investigate how can we prevent, treat and cure diseases, as well as how can we improve patient care.Why is it important that you consent?
We do medical research at Radboudumc. This research helps us develop new and improved treatments for future patients. read moreWhy is it important that you consent?
We do medical research at Radboudumc. This research helps us develop new and improved treatments for future patients.
If you consent, you help us to find out more about diseases and health conditions. We can also work on better diagnostics and treatment of future patients. For example, medical research teaches us how we can better prevent, treat and cure cancer and cancer-related conditions. We can also learn to improve quality of life. These findings are important for all patients.
How long is your consent valid?
If you consent to the use of your medical data and biomaterial for medical research, your consent will be valid until you withdraw it. Your consent also applies to all future medical data and biomaterial that we collect from you.What has been investigated in recent years?
We previously investigated how a specific genetic predisposition leads to the development of skin conditions, such as psoriasis and eczema. read moreWhat has been investigated in recent years?
We previously investigated how a specific genetic predisposition leads to the development of skin conditions, such as psoriasis and eczema. Thanks to research with biomaterial, researchers developed laboratory techniques to grow new skin. Using this "cultured skin", we can test the effect of a treatment. This has made some animal testing no longer necessary.
For some types of bladder cancer, the disease course cannot be predicted. Some patients worsen quickly, while other patients do not. In collaboration with a research group from Barcelona, we investigated why this happens. To find out how these patients differ from each other, we examined if they have unique features in their biomaterial. These findings help us better predict which patients will worsen quickly.
Your privacy is protected
We can only use your medical data and biomaterial for research if they have been numerically coded by your doctor. This ensures that a researcher cannot see which patient is involved. It also secures your privacy.FAQs
What kind of research uses medical data and leftover biomaterial?
Our researchers use medical data and biomaterial in various ways. For example, they look at differences in patient biomaterial and outcomes. They can also, for example, investigate whether a blood abnormality affects the occurrence or course of certain diseases. The researcher often also uses information from the medical file with the biomaterial.
Why is my biomaterial stored?
Blood, urine or tissue is used to determine why you are unwell. We store leftover biomaterial in case you return with new complaints. We can then use that biomaterial to investigate these complaints.
Medical research using your biomaterial is only conducted if there is enough leftover biomaterial. Tissue is always preserved for any necessary future diagnosis and treatment.
What are secondary findings?
If a researcher accidentally discovers a possible health concern during his or her research, we call this a secondary finding. The chance of a secondary finding is very rare. The researcher does not know that this finding is about you since your biomaterial is coded. This means that your medical data or biomaterial has a numerical code on it but not your name. If a possible health concern is accidently discovered, a special committee will assess whether it is important for you to know about it. If so, your treating doctor at the Radboudumc will determine which patient the biomaterial belongs to based on the coding. He or she will inform you and your primary health care provider.
Who are permitted to use your medical data and biomaterial?
- Doctors, researchers and medical interns in the Radboudumc
- Researchers in other healthcare institutions in the Netherlands collaborating with Radboudumc
- Researchers from other healthcare institutions abroad collaborating with Radboudumc
- Researchers from companies collaborating with Radboudumc
What are the rules and legislation regarding the use of medical data and remaining human material?
The Radboudumc follows rules established by doctors, researchers and patient associations. These rules are based on legislation. The most important rules are:
- The research must be useful and contributes to medical science.
- In some cases, the research will be evaluated by an ethical review committee.
- The research must have a small chance of secondary findings.
- Patient privacy must be appropriately protected.
We carefully handle your personal medical data. Please read below to see how we maintain your privacy.
We follow the rules and legislation for data processing and data security, such as the General Data Protection Regulation (GDP). We also follow medical research rules jointly developed by doctors, researchers and patient associations. These rules can be found in the "Good Use and Good Behavior Codes" (Federa-COREON).
- What happens if information from my patient file is needed for medical research?
Your medical data is coded to prevent a researcher from being able to identify you. The medical ethics review committee must approve all use of medical data and biomaterial for research.
- Do we collaborate with researchers outside the EU?
We will only share your data for medical research if they handle your data safely. We apply the data protection rules as agreed in the EU. We record these agreements in writing.
- Do you have more information on privacy?
You can read more about how we handle your data and biomaterial in our Privacy Statement. You can also learn about your rights as a patient. You can find our Privacy Statement at the bottom of this website.
How does consent work for minors or incapacitated patients?
Are you a parent/guardian of a child younger than 16 years old? If so, you can consent or object to the use of medical data and biomaterial on his/her behalf. However, children between 12 and 16 years can also object to the use of their medical data and biomaterials themselves.
Are you a legal representative of an incapacitated patient? An incapacitated patient refers to patient who cannot decide for him-/herself. For example, this may be someone with a severe intellectual disability or a person in a coma. If so, you can consent or object to the use of medical data and biomaterial on his/her behalf.
The law protects minors and incapacitated patients. The law states that medical data and biomaterial from these individuals may only be used if it is not possible to conduct the research without it.