
The way in which bone formation occurs needs to be redefined. This was revealed by Radboud university medical center researchers and their colleagues in a publication in Nature Communications. It turns out that bone formation does not require complex biomolecules in collagen at all. This means that the production of bone substitutes and biomaterials is less complicated than was previously thought.
Bones are vital. They form the scaffolding material for our muscles, which allow us to move. They protect vulnerable organs like the brain and lungs, and they accommodate the stem cells that produce our blood and immune system. Without bones, people could not exist. This is despite the fact that bones are seemingly very simple things, consisting mainly of a mix of collagen and calcium phosphate.
Orchestrating biomolecules?
Ten years ago, Nico Sommerdijk, theme Reconstructive and regenerative medicine, published an article in Nature Materials in which he and his colleagues demonstrated that nothing was needed other than collagen (long strands of connective tissue) and tiny pieces of calcium phosphate. “Nothing was known about the way in which the crystals in the collagen actually developed into small plates and then arranged themselves in an imbricate formation,” says Sommerdijk, who is a biochemical researcher at Radboud university medical center. “The generally accepted idea was that the bioactive molecules in this collagen strung the crystals together to form plates that were three nanometers thick, twenty nanometers wide and sixty-five nanometers long. A nanometer is one billionth of a meter.”
Ten years ago, it was not possible to depict these processes on such a tiny scale. But Sommerdijk soon came to doubt the idea that had been embraced by his field. Sommerdijk: “When we replaced the calcium phosphate with other minerals, the same tiny plates were created. Bioactive molecules are very selective, which immediately made us question the prevailing idea.” What followed was a scientific Sisyphean task that was carried out against the establishment, which after ten years has now ruled in his favor with a publication in Nature Communications.
Going against the tide
The first thing that Sommerdijk challenged was the idea of crystallized plates being stacked ‘like a deck of cards’. Using an electron microscope he recorded approximately one hundred two-dimensional (2D) images of bone under different angles, which resulted in three-dimensional images (3D) that showed something entirely different. Even though the plates were all laying in the longitudinal direction of the collagen, they were not neatly stacked against each other like playing cards. The new plates that could continuously be seen lay against each other at different angles.
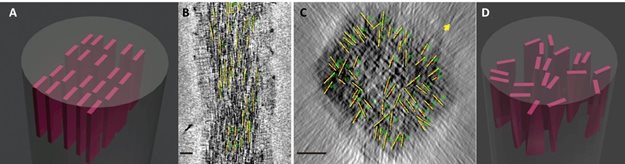
The arrangement of mineral crystals in bone. A) The old model with all of the crystals lined up ‘like a deck of cards’, B & C) Electron microscope recordings of the bone crystals in the collagen showing the crystals in the longitudinal direction (B) and the crystals in the transverse direction (C) and D) The new model showing crystals that are only longitudinally aligned.
Together with the expert who first reported on the structure of collagen in 2006 and a specialist who studies crystallization in confined spaces, the function of the collagen was meticulously investigated. Collagen appeared to have narrow cavities in the length direction, which were infiltrated by the calcium phosphate that then started to crystallize. “This wasn’t happening under the guidance of bioactive molecules in the collagen, but was a blind process that was dominated by the laws of physical chemistry”, says Sommerdijk. “Calcium phosphate that crystallized in the length direction had a selection advantage, precisely because of these narrow collagen cavities. As a result, it was only very thin needles that were initially created in such a cavity. This needle then grew out into a small plate of calcium phosphate that pushed the surrounding collagen away.”
The basis of bone formation
It is not possible to closely monitor the process of bone growth in humans. Sommerdijk, who works closely with cell biologist Anat Akiva at Radboud university medical center, has been using a model in which bone growth can be simulated outside an organism. This allowes him to see the process as it gradually takes place. And the final result is similar to what he has found in the human bone material that has frequently become available after surgery. “This has consequently allowed us to gradually provide a new argumentation for the way in which our bones are formed, and how they grow and harden.”
This fundamental research also has consequences from the practical viewpoint, claims Akiva. “If the growth of crystals need not take place in a matrix with extremely complex and precious biomolecules, but can also simply be done in channels made of inexpensive polymer, this will drastically simplify the process of making biomaterials and bone substitutes. And if we can do this without having to sacrifice the properties of these materials it means that our results will also be valuable to the patient.”
ERC Advanced Grant
The article in Nature Communications will need to overturn the perception of bone formation once and for all. But the process of bone formation is more complex than this, claims Sommerdijk: “Bone is not only formed within, but also outside the collagen. Various proteins seem to play a role in this process.” Sommerdijk will be using an ERC Advanced Grant of 3.5 million euros that he previously received in order to gain more insight into this.
Related news items
Self-healing injectable hydrogels: an emerging class of novel biomaterials
30 August 2022Researchers from Radboudumc and Harvard University have jointly published a review article on self-healing injectable hydrogels in Chemical Reviews.
go to page.aspx?width=439&height=263&ext=.png&type=BlockColumn1Zoom1)
Vote for the RIMLS Supervisor of the year
7 December 2021This is your chance to vote for your favourite candidate for supervisor of the year! Support and promote your ideal supervisor from the three nominees: Siroon Bekkering, Gerald Verhaegh and Jo Zhou. Please submit your vote the 20th of December 2021 at the latest.
go to page
Willeke Daamen ratified as ETRS board member
3 November 2021 Willeke Daamen, theme Reconstructive and regenerative medicine appointed as board member of The European Tissue Repair Society (ETRS). go to page
RIMLS awards call for nominations
19 October 2021 RIMLS awards several prizes to stimulate and honor our (young) researchers. Upcoming awards are Supervisor of the Year, Best Master Thesis, Best Publication, Best Image and more. Send your nominations now before 24 November 2021. go to page
Turbo Grants for four medical-technical research projects
19 October 2021 Four TURBO grants were recently awarded to new technical-medical research proposals. The grants are part of the TURBO program, a collaboration between the University of Twente (TechMed Centre) and the Radboudumc. go to page
